iPSC-derived MASH model
Our in vitro MASH co-culture model represents an optimized cell platform for drug discovery applications and a principal tool for elucidating the underlying mechanisms of the disease
Metabolic Dysfunction–Associated Steatohepatitis (MASH)
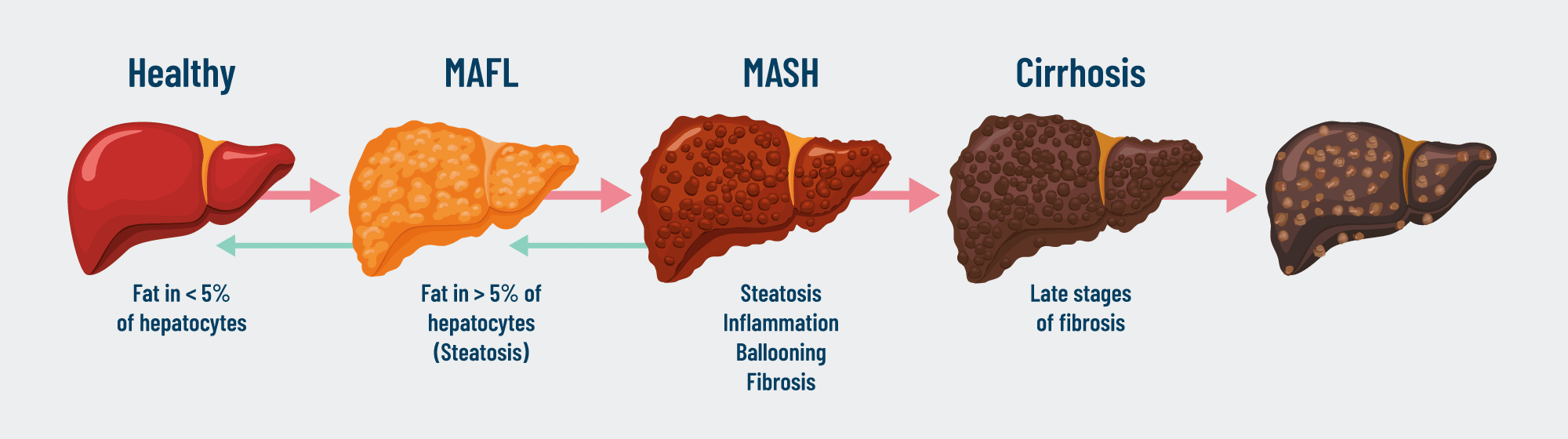
MASH is a progressive liver disease characterized by fat accumulation, inflammation, fibrosis and liver cell damage. It is the advanced form of MASLD (Metabolic dysfunction–associated steatotic liver disease) and can lead to cirrhosis, and eventually to the development of hepatocellular carcinoma.
Using our wild-type iPSC-derived hepatocytes (Ulti-HEP) and wild-type iPSC-derived Hepatic Stellate cells (Ulti-HSC), we have developed a novel in vitro MASH co-culture model that recapitulates the disease phenotype in-a-dish, offering an optimized model for drug discovery applications and a principal tool for elucidating the underlying mechanisms of the disease.

Limitations in animal and primary cell models
Currently, researchers rely on spheroid models or liver-on-a-chip models consisting of primary human hepatocytes and primary human hepatic stellate cells. However, these in vitro models come with significant drawbacks.
Significant donor-to-donor variability due to limited availability
Current models, such as primary cell systems, rely on cells isolated from multiple donors, introducing variability that undermines data reproducibility.
Hepatic Stellate cells are not quiescent
Primary human stellate cells are difficult to source, given they make up less than 10% of the liver. During cell culture they are already activated, or possibly a collection of differentially activated stellate cells. This activation further compromises reproducibility downstream.

Compromised health of cells introduces variability
Current MASH models usually come from resected human liver tissues that are already diseased. These "healthy parts" of liver are from patients with liver cancer, or from donors that have been exposed to a series of pharmacological, dietary interventions, or alcohol consumption.
These cells may already exhibit altered metabolic function, stress responses, or gene expression patterns before any experimental variables are introduced, making it difficult to:
- Establish a reliable baseline for comparison.
- Accurately model disease progression.
- Interpret the true impact of candidate therapeutics.
- Ensure reproducibility across studies.
Key Features of the MASH co-culture model
Our MASH co-culture model de-risks drug discovery by providing a new and highly predictive in vitro screening platform suitable for high throughput studies.
Human-Relevant, Isogenic Co-Culture Platform
Our highly functional co-culture system consists of iPSC-derived hepatocytes and quiescent hepatic stellate cells from the same genetic background, eliminating inter-donor variability and enabling consistent, reproducible results.
Healthy, Stable, and Scalable Cells
Unlike primary cells, DefiniGEN’s hepatocytes are free from pre-existing lipid accumulation or inflammation and maintain full functionality in a scalable 2D platform—offering a robust alternative to variable 3D models.
Versatile and Disease-Responsive
The co-culture accurately models steatosis and fibrosis through dietary and pharmacological triggers, generating measurable endpoints such as lipid accumulation and collagen secretion—ideal for MASH research and therapeutic development.
Genetically Customisable for Mechanistic Insights
Use CRISPR-edited or patient-derived iPSCs to explore MASLD-linked genetic variants (e.g., PNPLA3, TM6SF2), supporting advanced research into disease mechanisms and personalized medicine approaches.
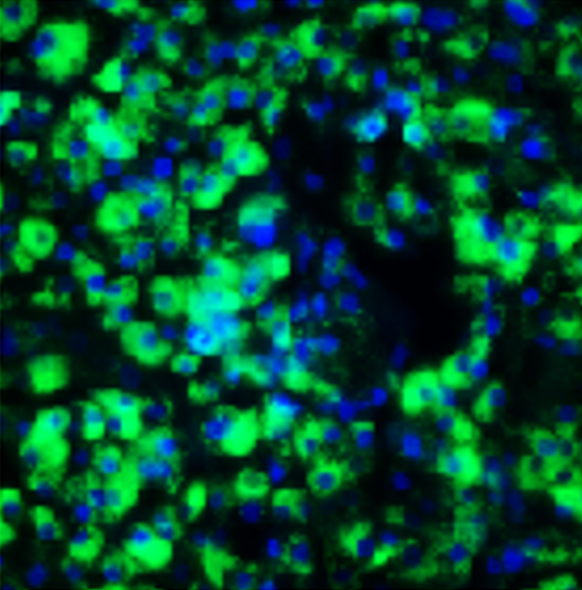
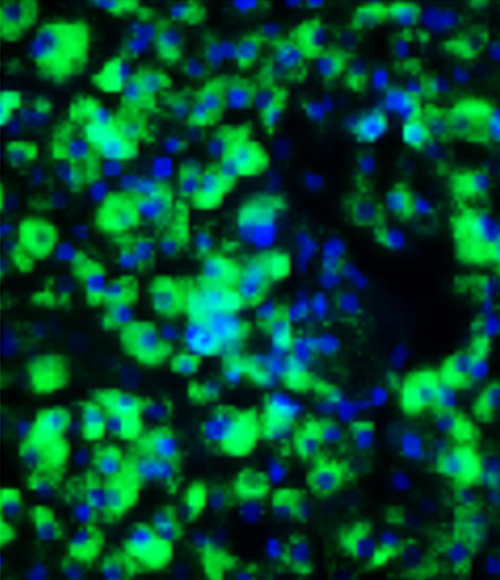
Our MASH co-culture model is phenotypically relevant
When our co-culture is treated with free fatty acids, they demonstrate increased lipid accumulation and collagen secretion. Additionally, the model can be treated pharmacologically to reverse these effects. This makes the co-culture model an accurate, human derived model to screen compounds in high throughput investigations.
iPSC-derived Ulti-HEP and Ulti-HSC can be successfully co-cultured


Ulti-HEP/HSC co-cultures express hepatocyte and stellate cell markers

DefiniGEN Ulti-HEP, Ulti-HSC, and Ulti-HEP/HSC co-cultures.

GFAP and hepatocyte marker albumin (ALB) in in DefiniGEN Ulti-HEP, Ulti-HSC, and Ulti-HEP/HSC co-cultures. Data are presented as mean±SEM of n=2 independent
experiments. mRNA expression data were normalized to 18S rRNA.
Activated Ulti-HSC drive hepatic steatosis in Ulti-HEP/HSC co-cultures
and this can be pharmacologically reversed


Our MASH co-culture secretes collagen, and this can be pharmacologically
reversed

Fatty acid treatment drives steatosis in Ulti-HEP/HSC co-cultures, and
this can be pharmacologically reversed

BODIPY staining.

Free Fatty Acid treated Ulti-HEP/HSC co-cultures secrete collagen, and this can be pharmacologically reversed


Ready to get the most out of your MASH investigation?
Contact us to discuss your project with one of our experts

