Publication
Intestinal Monolayer
DefiniGEN’s intestinal monolayer product INTReady constitutes a ground-breaking new system to model the permeability of the human intestine and investigate drug efflux.
Physiologically relevant.
DefiniGEN’s intestinal monolayer product INTReady constitutes a ground-breaking new system to model the permeability of the human intestine and investigate drug efflux. The cells are grown as a monolayer in a similar manner to standard Caco-2 immortalized cell lines with the key benefit of greatly increased biological relevance, as the cells have primary human intestinal cell function, metabolism and genetics.
Donor background
Wild-type donor genetics and karyotype verified
Applications
Cells can be used to model the permeability of the human intestine and investigate drug efflux
Key gut markers
Display multiple key gut markers OLFM4, CHGA, MUC2, Villin and KRT19
Culture Conditions
Monolayer format grown in a similar manner to standard Caco-2 immortalized cell lines
Monolayer culture
Monolayer format grown in a similar manner to standard Caco-2 immortalized cell lines
Technical Data
Monolayer formation
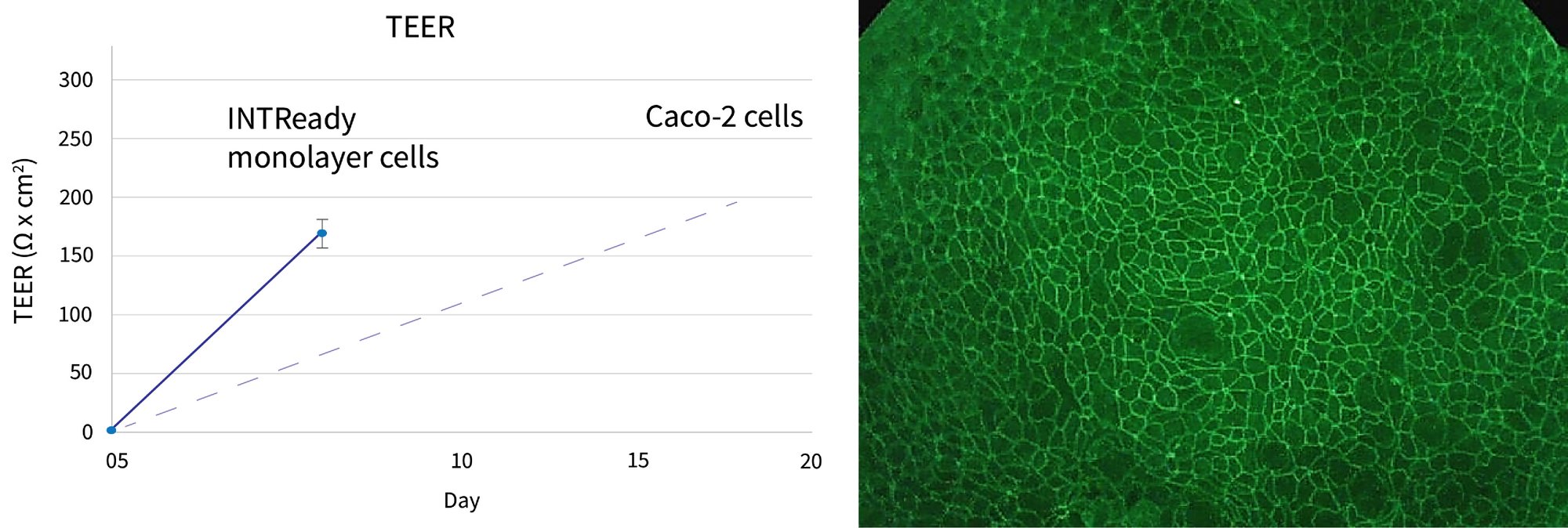
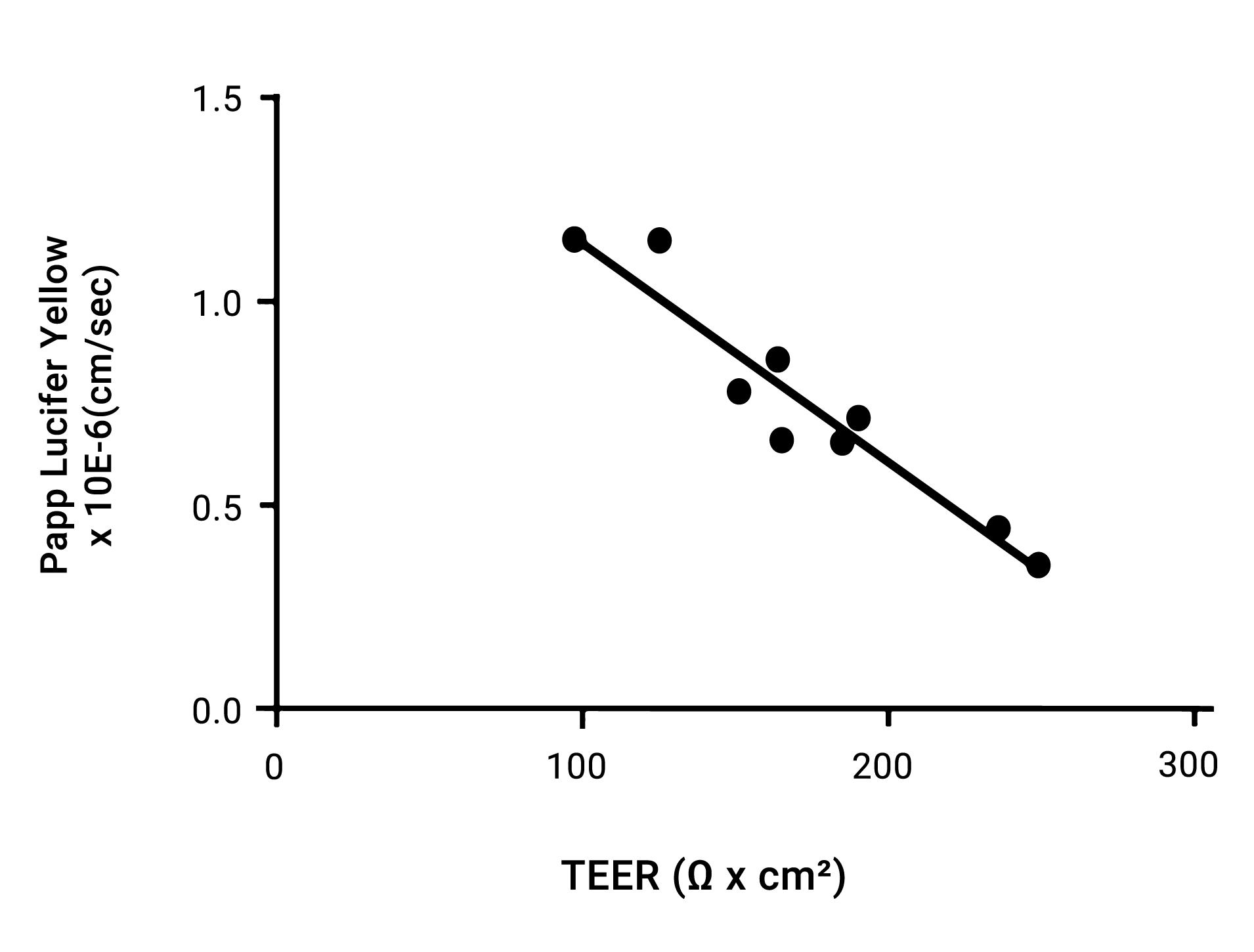
Confluent monolayer formation has been demonstrated via TEER, (transepithelial electrical resistance), ZO1 (Zonula Occludens/Tight Junction Protein-1) immunostaining, and Lucifer Yellow analysis. TEER measurement of the monolayer demonstrates tight junction formation with typical values of >150ohm x cm2 being observed after 7 days of monolayer culture. These levels are comparable to those observed with a reference Caco-2 line after 20+ days. ZO1 protein localization immunostaining at the cell boundaries of all cells analyzed indicates a confluent monolayer has been formed. Correspondingly, Lucifer Yellow assays to assess the permeability characteristics of the monolayer has established monolayer integrity with observed values of Papp = 0.87 ± 0.32 × 10-6 cm/sec (A to B, 100 μM, 60 min).
Figure 1. Verification of intestinal cell monolayer integrity. (A) TEER measurement of the INTReady monolayer demonstrates tight junction formation with typical values of >150ohm x cm2 being observed after 7 days of monolayer culture. (B) ZO1 localization at the cell boundaries signifies the presence of tight junctions in the monolayer.
Intestinal permeability
Figure 2. Def-INT Ready permeability assessment. Monolayer evaluation is consistent when using both TEER measurements and checking permeability of low permeability compounds such as Lucifer Yellow.
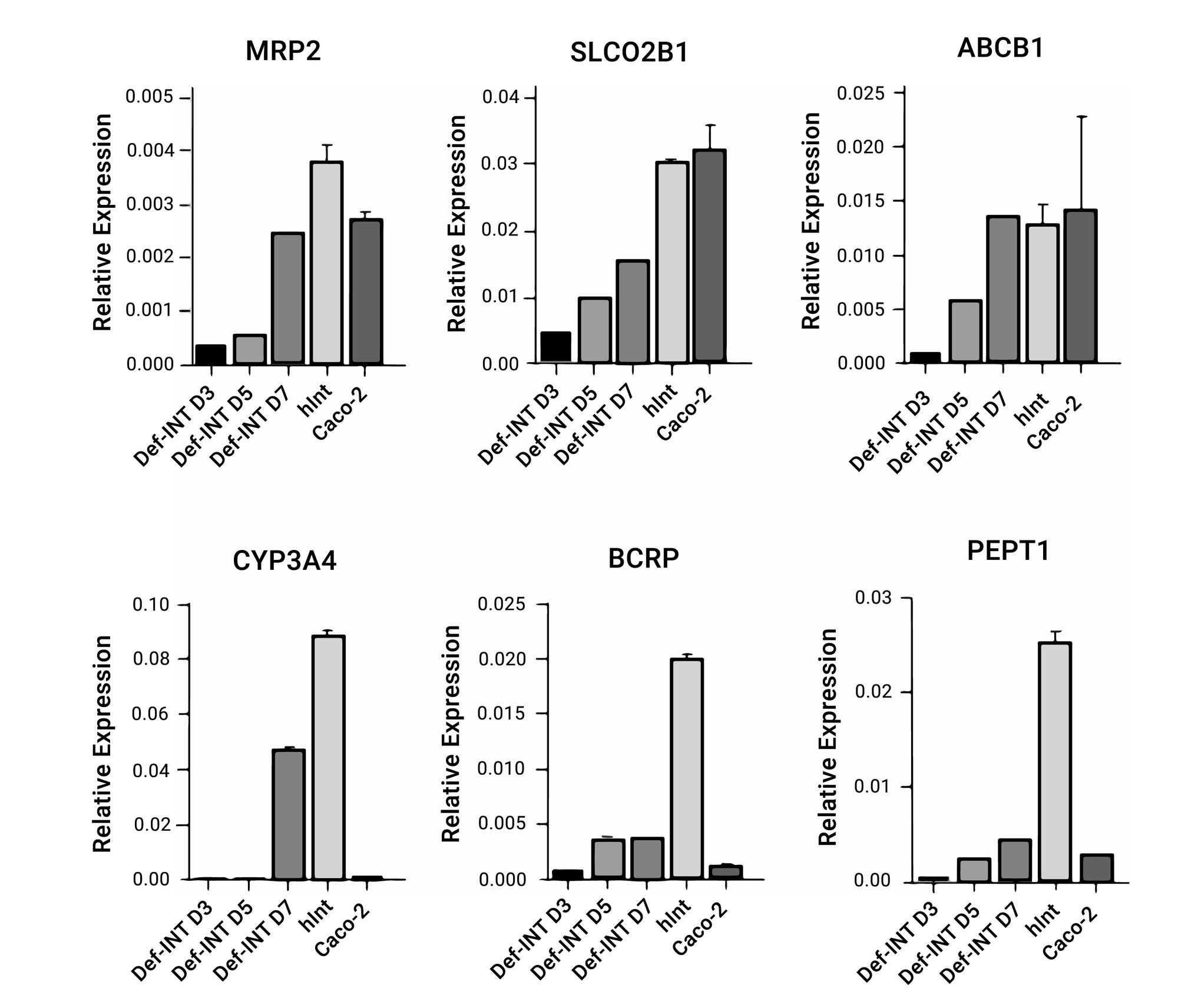
Biologically relevant system
Figure 3. Def-INTESTINAL monolayer cells express similar gene expression profiles to primary human intestinal cells.
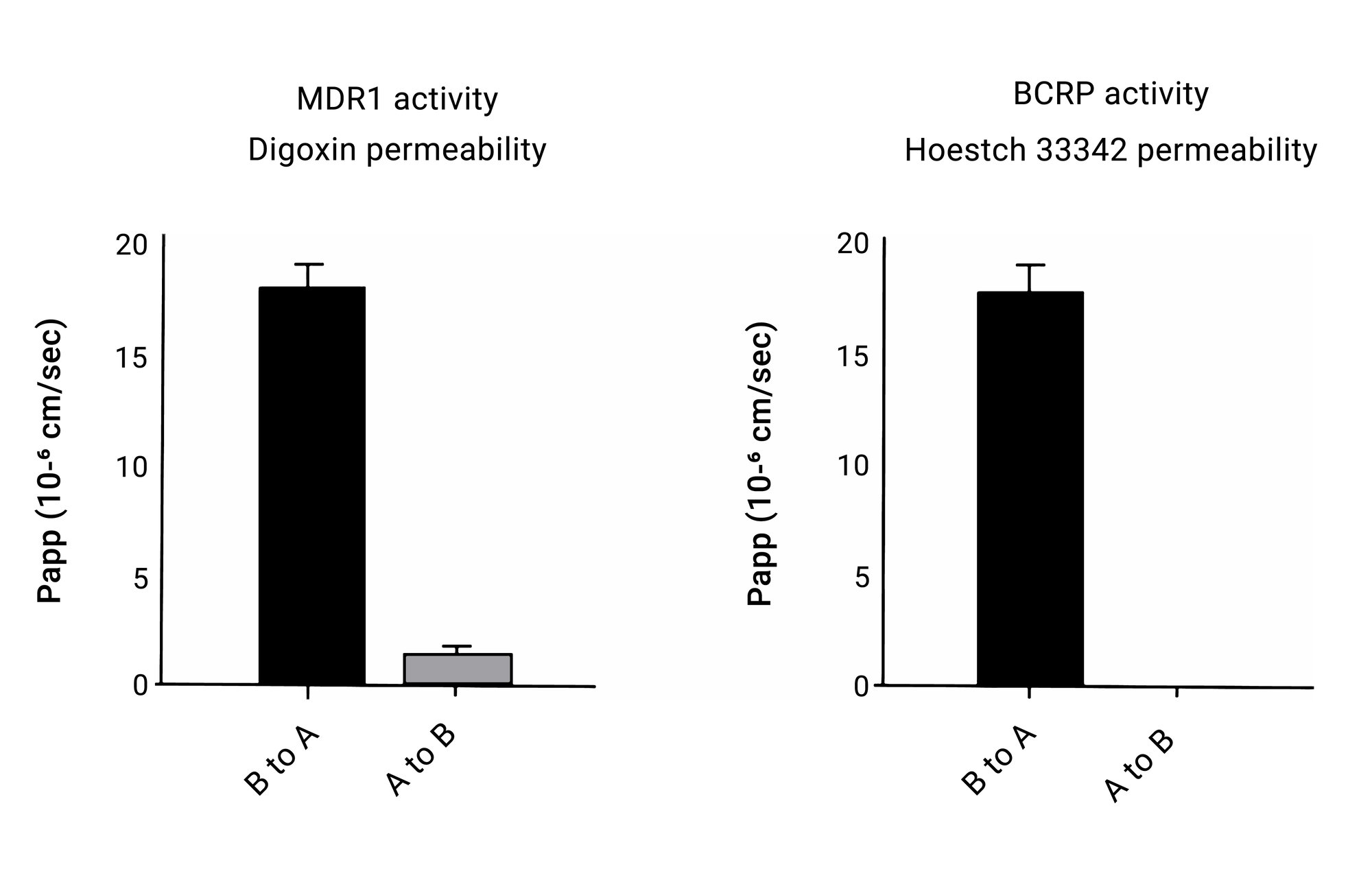
Efflux transporter activities
Figure 4. Functional ABCB1 (MDR1) and BCRP transporter activity. INTReady monolayer cells exhibit functional efflux transporter activities when Digoxin and Hoechst 33324 were used as ABCB1 (A) and BCRP substrates respectively (B). These results indicate the presence of a polarized monolayer with functional apical transporter activities being observed.
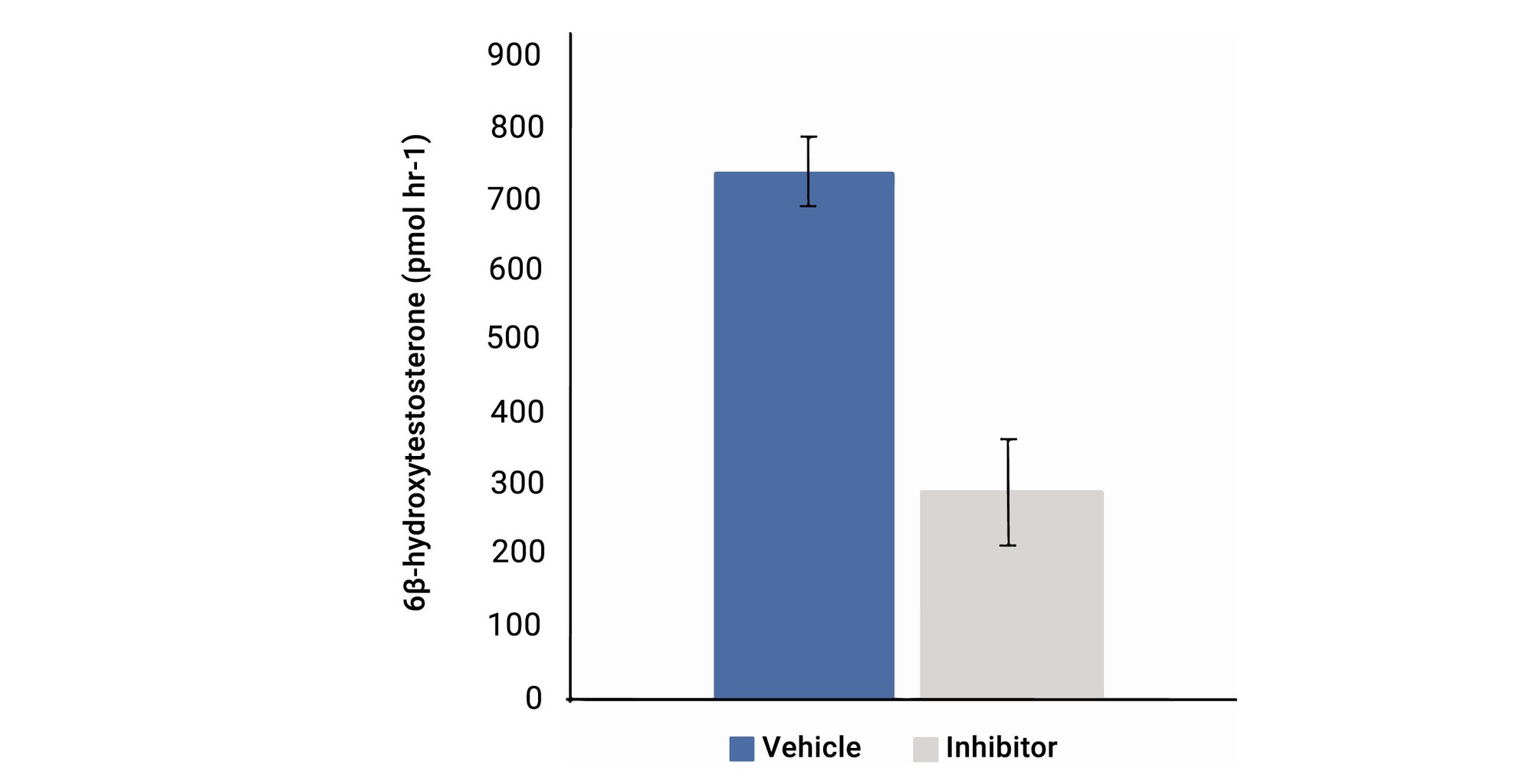
CYP3A4 activity assay
Figure 5. CYP3A4 activity was measured in the Def-INT monolayer via their ability to hydrolyze testosterone into 6B-hydroxytestosterone. This activity was reduced to less than 40% derivative in the presence of a recognized inhibitor Ketoconazole.
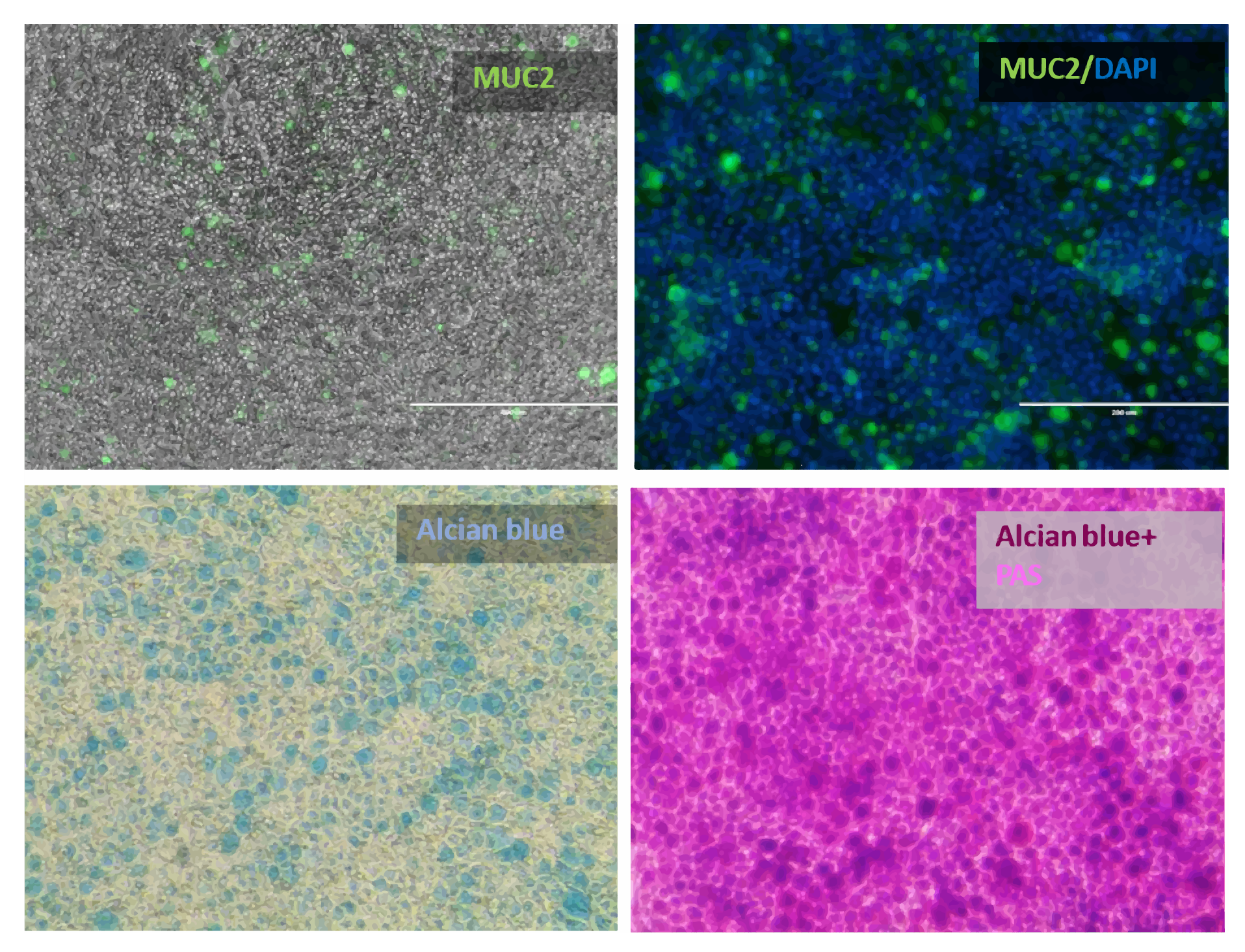
Def-INT monolayer secretes mucus-forming proteins
Figure 6. Def-INT monolayer secretes mucus-forming proteins making it a more physiological model. Immunofluorescence shows presence of MUC2, a specific marker for goblet cells. Alcian blue staining demonstrates the presence of acid mucins while PAS staining shows presence of both acid (dark purple) and neutral mucins (bright magenta).
Drug permeability
For validation, a set of compounds were screened with Caco-2 and Def-INT cells grown in monolayer. Permeability assay over a range of permeabilities show a good correlation with Caco-2. Def-INT grown monolayer shows better predictability for Furosemide than Caco-2.
Transepithelial electrical resistance analysis
.png?width=2000&name=DefiniGEN_intestinal_monolayer_TEER_analysis%20(1).png)
Key Publications
Publication
Interaction of Salmonella enterica Serovar Typhimurium with Intestinal Organoids Derived from Human Induced Pluripotent Stem Cells
Publication


