Predicting Hepatotoxicity
Hepatically relevant model providing robust human data.
Drug-Induced Liver Injury (DILI) predictivity
Drug-induced Liver Injury (DILI) is a leading cause for drug failure in clinic, accounting for more than 50% of liver failure cases. DILI is mainly the result of poor suitability of the available pre-clinical models to predict toxicity of positive compounds, either due to species-specific differences (e.g., animal models, animal-derived cell lines), donor-to-donor variability and short-life span (e.g., primary human hepatocytes), or low metabolic capacity (e.g., hepatocellular carcinoma cell lines). Recently, efforts to address the short-life span of primary human hepatocytes have resulted to the generation of 3D in vitro liver models with promising results. However, these models still fail to address the donor-to-donor variability issue, limiting their suitability for high-throughput screening during the early phases of the discovery process.
Here, we assessed DefiniGEN’s Opti-HEP ability to accurately predict toxicity risk for a broad set of compounds with known DILI liability. The results revealed that Opti-HEP can accurately predict DILI across a dose-response using cell viability (ATP content) as endpoint, consistent with the functional CYP450 enzyme expression and activity demonstrated below (Figure 1). These data alongside the expansion capacity and lack of donor-to-donor variability iPSC-derived cell models offer highlight both the superiority of Opti-HEP over the current hepatocyte models and their suitability in high-throughput hepatotoxicity screening.
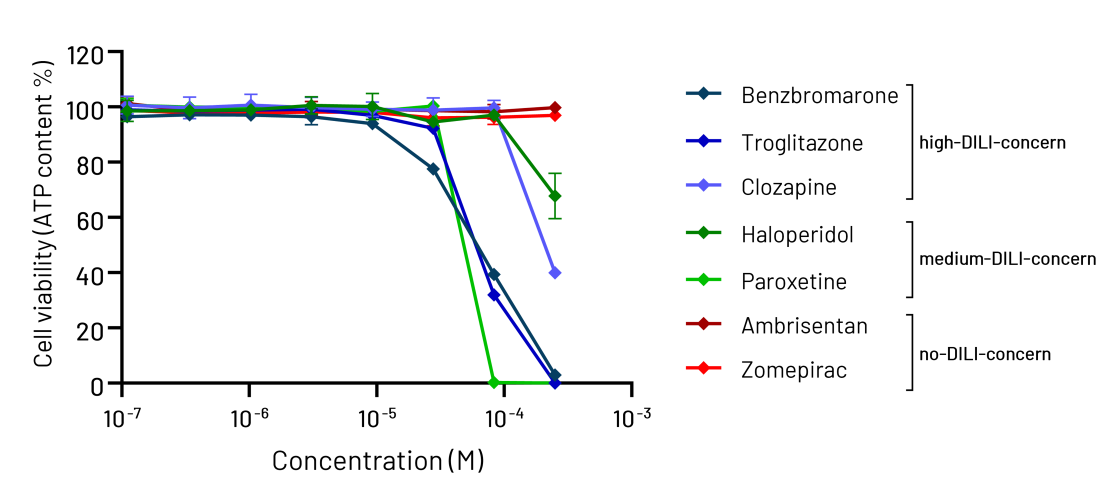
Figure 1: Cell viability (ATP content) in DefiniGEN Opti-HEP following 48h of treatment with increasing concentrations (0-250 μM) of compounds with known DILI liability (classification based on Proctor et al. 2017). Cell viability data were normalised to positive (250 μM chlorpromazine) and negative controls (0.2% DMSO) and are presented as mean±SD of n=3 technical replicates.
It should be noted that, in addition to the endpoint assays showed above, our cells can be used for the measurement of additional endpoint clinical biomarkers that are routinely tested during DILI screening, including albumin, urea, and ALT (data not shown).
DefiniGEN’s Opti-HEP offer an improved in vitro liver platform for high-throughput ADME and toxicology screening, aiming to protect patients’ lives, minimise risks while reducing costs, and pave the way for a more efficient and effective future in the field of drug discovery. Please contact our scientists for additional information.

